
Biorigin
Savory Flavor Enhancers, Flavors and Mold Inhibitors.
Catalog
Documents
-
Allergens
(visible)
Click to download A -
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Nutrition
(not visible) N -
Suitability requirements
(not added) SR
natural flavor
Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Nutrition
(not visible) N -
COA template
(not added) COA -
Item questionnaire
(not added) IQ -
Suitability requirements
(not added) SR
Natural flavor made from cultured whey.
Documents
-
Allergens
(visible)
Click to download A -
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not added) N
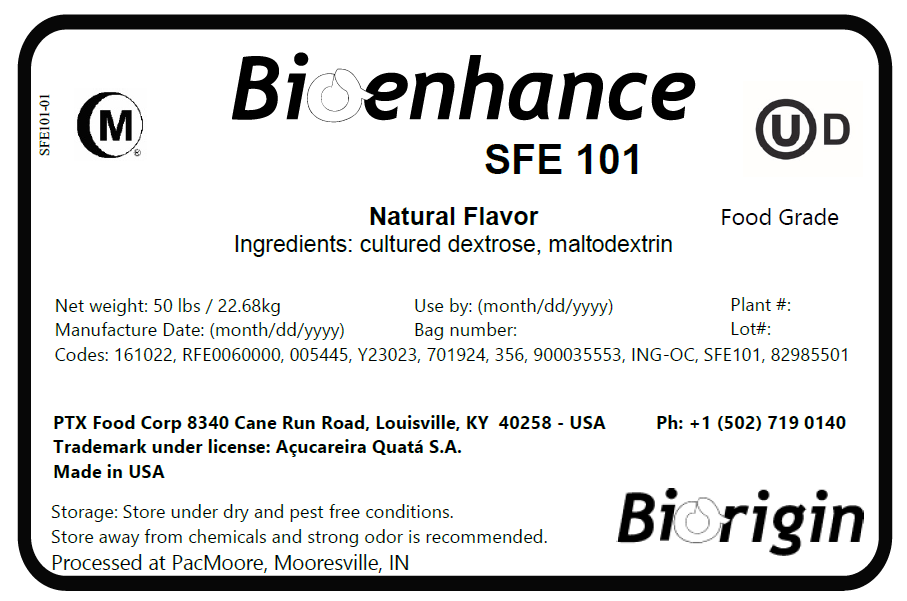
BIOENHANCE SFE 101 is a natural flavor produced by
dextrose fermentation. It has a very clean savory flavor, provides
flavor enhancement while maintaining integrity of the original
flavor profile. It is also excellent for use in or in conjunction
with natural salt replacer systems, due to its clean savory flavor
profile. It is a spray dried powder with a tan to golden tan
color.
Documents
Documents
-
Allergens
(visible)
Click to download A -
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not added) N
Documents
-
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not visible) N -
Allergens
(not added) A
Low sodium natural flavor produced through fermentation.
Documents
Natural ultra low sodium flavor enhancer in custom size.
Documents
-
COA template
(visible)
Click to download COA -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not visible) N -
Allergens
(not added) A -
Country of origin
(not added) COO -
Item questionnaire
(not added) IQ
Natural flavor designed to improver overall product quality and extend product shelf life.
Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
COA template
(not added) COA -
Nutrition
(not added) N
Documents
It is a yeast extract produced from Saccharomyces cerevisiae. It has a brown color with a dark roasted meat taste profile. It contains a high level of natural nucleotides that brings out a typical overall savoury taste profile, blowing up the Umami effect. It is a free flowing agglomerated powder and instantly water soluble. It's 100% natural and Non GMO.
Documents
-
Allergens
(visible)
Click to download A -
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not visible) N
Documents
-
Allergens
(visible)
Click to download A -
COA template
(visible)
Click to download COA -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
SDS (Safety Data Sheet)
(visible)
Click to download SDS -
Suitability requirements
(visible)
Click to download SR -
Nutrition
(not visible) N
It is a inactive dry yeast produced from Saccharomyces cerevisiae specially selected to be used in the food and pharmacy industry. It is a fine free flowing powder, spray dryed, of a light beige color with a smooth savoury character. It contains glutathione and high quality protein, minerals, dietary fibers and B vitamins. It is 100% natural and Non GMO.
Locations
| Location name | Address |
|---|---|
| Biorigin Brazil | Fazenda Quata S/N Quata, SP 19789-899 BRA |
| Biorigin PTX Food Corporation | 8340 Cane Run Road Louisville, KY 40258 USA |
| Biorigin S.A. | FAZENDA QUATÁ S/N QUATA, SP 19780-000 BRA |
Documents
| Type | Location |
|---|---|
| PC Allergen Control Policy | Biorigin PTX Food Corporation |
| Food Fraud Mitigation Strategies | Biorigin PTX Food Corporation |
| PC HACCP / Food Safety Plan (Facility) | Biorigin PTX Food Corporation |
| Recall Plan | Biorigin PTX Food Corporation |
| Traceability | Biorigin PTX Food Corporation |
| BSE Statement | Biorigin Brazil |
| Recall Plan | Biorigin Brazil |
| Lot Code | Biorigin Brazil |
| Allergen Control Policy | Biorigin Brazil |
| Allergen Control Policy | Biorigin PTX Food Corporation |
| CTPAT | Biorigin Brazil |
| Supplier Approval Program Statement | Biorigin PTX Food Corporation |
| Traceability | Biorigin Brazil |
| CA Transparency Act | Biorigin Brazil |
| Foreign Supplier Verification Prgm | Biorigin Brazil |
| Supplier Approval Program Statement | Biorigin Brazil |
| Recall/Emergency/Contact List | Biorigin Brazil |
| GFSI Certificate | Biorigin PTX Food Corporation |
| 3rd Party Audit Corrective Action Plan | Biorigin PTX Food Corporation |
| Receipt Acknowledgement | Biorigin PTX Food Corporation |
| Operating License | Biorigin Brazil |
| 3rd Party Audit Report | Biorigin PTX Food Corporation |
| GFSI Corrective Action | Biorigin PTX Food Corporation |
| GFSI Audit Report | Biorigin PTX Food Corporation |
| GFSI Audit Report | Biorigin Brazil |
| 3rd Party Audit Report | Biorigin Brazil |
| GFSI Corrective Action | Biorigin Brazil |
| 3rd Party Audit Report | Biorigin S.A. |
| 3rd Party Audit Certificate | Biorigin S.A. |
| 3rd Party Audit Certificate | Biorigin Brazil |
| GFSI Certificate | Biorigin Brazil |
| 3rd Party Audit Corrective Action Plan | Biorigin Brazil |
| CHG Supplier Food Safety & Quality Expectations Manual Acknowledgment | Biorigin Brazil |
| Chlorpyrifos Statement | Biorigin PTX Food Corporation |
| Ethical Code of Conduct | Biorigin Brazil |
| Contact Profile | Biorigin PTX Food Corporation |
| Letter of Guarantee | Biorigin PTX Food Corporation |
| Environmental Questionnaire | Biorigin PTX Food Corporation |
| Evidence of Auditor's Qualifications | Biorigin PTX Food Corporation |
| Fire Department Inspection | Biorigin Brazil |
| Bioterrorism Letter | Biorigin Brazil |
| Health License | Biorigin Brazil |
| FDA Registration | Biorigin Brazil |
| FDA Registration | Biorigin PTX Food Corporation |
| HACCP Plan (Facility) | Biorigin Brazil |
| HACCP/Hazard Analysis/Food Safety Plan | Biorigin Brazil |
| Food Defense Plan Statement | Biorigin PTX Food Corporation |
| FDA Registration or USDA Registration & Plant ID | Biorigin Brazil |
| HACCP Plan (Facility) | Biorigin PTX Food Corporation |
| Supply Chain PC Questionnaire (FSQS 4.3.3) | Biorigin PTX Food Corporation |
| Letter of Guarantee | Biorigin Brazil |
| Halal - PPT | Biorigin PTX Food Corporation |
| Insurance | Biorigin PTX Food Corporation |
| Insurance | Biorigin Brazil |
| Environmental Policy | Biorigin Brazil |
| Environmental License | Biorigin Brazil |
| Lot Code | Biorigin PTX Food Corporation |
| 3rd Party Audit Certificate | Biorigin PTX Food Corporation |
| Bioterrorism Letter | Biorigin PTX Food Corporation |
| CA Transparency Act | Biorigin PTX Food Corporation |
| CTPAT | Biorigin PTX Food Corporation |
| W-9 | Biorigin Brazil |
| Recall/Emergency/Contact List | Biorigin PTX Food Corporation |
| Chemical Hazards - Sweetener Questionnaire | Biorigin PTX Food Corporation |
| Supplier Questionnaire - Addendum | Biorigin PTX Food Corporation |
| Supplier Questionnaire EU/UK | Biorigin S.A. |
| Supplier Questionnaire | Biorigin PTX Food Corporation |
| Supplier Questionnaire | Biorigin Brazil |
| Supplier Expectations and Requirements 10.1.1 | Biorigin Brazil |
| Sustainability (Level 1) - Addendum | Biorigin Brazil |
| Sustainability (Level 1) | Biorigin PTX Food Corporation |
| Sustainability (Level 1) | Biorigin Brazil |
| Sustainability (Level 2) - Addendum | Biorigin Brazil |
| Sustainability (Level 2) | Biorigin PTX Food Corporation |
| Sustainability (Level 2) | Biorigin Brazil |
| Food Defense Plan Statement | Biorigin Brazil |
| Food Fraud Mitigation Strategies | Biorigin Brazil |
| W-9 | Biorigin PTX Food Corporation |
| Environmental Policy | Biorigin PTX Food Corporation |
| Ethical Code of Conduct | Biorigin PTX Food Corporation |