
Kwality Labels INC
Flexo & Digital Print for Packaging
Industry recognized certifications:
FTA FIRST – Flexographic Image Reproduction Specifications & Tolerances
G7 master color managed workflow
IFS HACCP for food safety and quality standards
Servicing Canada, US, and the Global Market
Documents Up to Date
Catalog
Documents
Documents Up to Date

Documents
Cold foil is an on-press application of a thin layer of foil onto the label substrate commonly seen in the beverage & beauty industries.
Wine, beer, and spirits’ producers are in a crowded trillion dollar marketplace and face an on-going challenge in winning market shares.
Use textured stock with cold foil to create a metallic, high-end, sophisticated, dramatic look.
Silver, gold or any other shiny tint will draw attention to a compelling label that helps connect with the buyer.
Documents Up to Date
Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Nutrition
(visible)
Click to download N -
Suitability requirements
(visible)
Click to download SR -
COA template
(not added) COA -
Product specification
(not added) PS -
SDS (Safety Data Sheet)
(not added) SDS
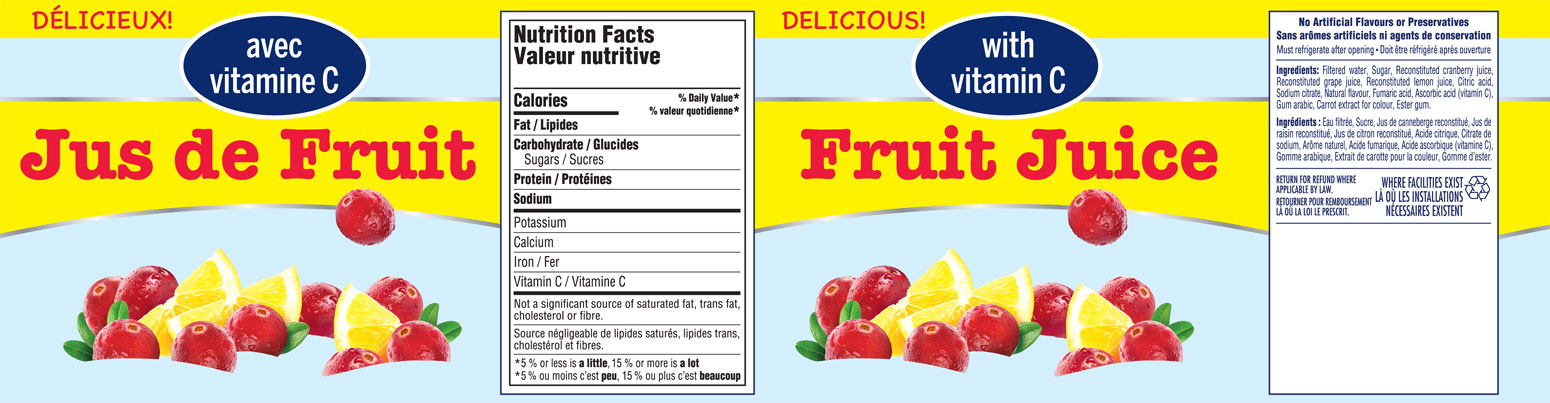
Cut & Stack/Sheeted labels are die cut to size with or without backing (supported/unsupported) and stacked to a required bundle size.
These labels can be wrapped around products that may be glued with an overlap to achieve a 360° branding.
Alternatively, they may be glued onto the product directly, for instance, a juice bottle.
Documents Up to Date
Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Nutrition
(visible)
Click to download N -
Suitability requirements
(visible)
Click to download SR -
COA template
(not added) COA -
Product specification
(not added) PS -
SDS (Safety Data Sheet)
(not added) SDS
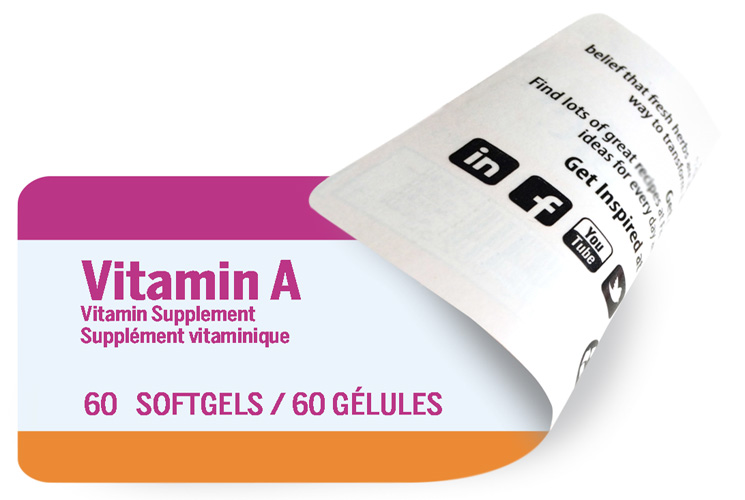
Sometimes called extended content, these types of labels are used to increase real estate within a small space.
Printed front and back to convey extensive information commonly seen with vitamin, nutraceutical and chemical products.
These labels may be hinged or multi-layered with a smooth peel back to reveal more content.
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date

Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Nutrition
(visible)
Click to download N -
Suitability requirements
(visible)
Click to download SR -
COA template
(not added) COA -
Product specification
(not added) PS -
SDS (Safety Data Sheet)
(not added) SDS
Pressure sensitive, also known as stickers, can be printed on paper, film or foil.
This solution can be applied to almost any package and is by far the most common choice for clients including thermal transfer and direct thermal applications.
The label can be peeled off the backing and lightly applied by hand or alternatively machine applied.
Adhesion can be removable or permanent and last through high or low temperatures depending on the requirements.
We provide blanks or pre-printed labels for your printers.
Documents Up to Date

Documents
Kwality Labels is the leader in the spiral label market in Canada, producing labels for high quality packaging within the food and beverage industries including chip/snack canisters and frozen juice containers.
We also produce a variety of spiral applications, for example,
caulking tubes and tape cores.
These spiral wound labels, that wrap around composite materials that form the canister, can showcase impactful graphics top to bottom.
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
-
Allergens
(visible)
Click to download A -
Country of origin
(visible)
Click to download COO -
Item questionnaire
(visible)
Click to download IQ -
Product specification
(visible)
Click to download PS -
COA template
(not added) COA -
Nutrition
(not added) N -
SDS (Safety Data Sheet)
(not added) SDS -
Suitability requirements
(not added) SR
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Documents
Documents Up to Date
Locations
| Location name | Address |
|---|---|
| Kwality Labels INC - Corporate | 563 Edward Ave Unit #8 Richmond Hill, ON L4C9W8 CAN |
Documents
| Type | Location |
|---|---|
| Supplier Questionnaire | Kwality Labels INC - Corporate |
| Organizational Chart | Kwality Labels INC - Corporate |
| Recall Plan | Kwality Labels INC - Corporate |
| Quality Manual | Kwality Labels INC - Corporate |
| Environmental Monitoring SOP | Kwality Labels INC - Corporate |
| Environmental Policy | Kwality Labels INC - Corporate |
| Audit report | Kwality Labels INC - Corporate |
| 3rd Party Audit Corrective Action Plan | Kwality Labels INC - Corporate |
| 3rd Party Audit Report | Kwality Labels INC - Corporate |
| 3rd Party Audit Certificate | Kwality Labels INC - Corporate |
| Supplier Contact Sheet | Kwality Labels INC - Corporate |
| Continuing Letter of Guarantee | Kwality Labels INC - Corporate |
| Copies of FDA Establishment Inspection Reports, Form 483s and/or warning letters received | Kwality Labels INC - Corporate |
| FDA Registration | Kwality Labels INC - Corporate |
| Supplier/Contract Manufacturer Qualification Questionnaire | Kwality Labels INC - Corporate |
| Recall/Emergency/Contact List | Kwality Labels INC - Corporate |
| Insurance | Kwality Labels INC - Corporate |
| Letter of Guarantee | Kwality Labels INC - Corporate |
| Supplier Manual Acknowledgment | Kwality Labels INC - Corporate |
| Shearer's Foods Supplier Manual | Kwality Labels INC - Corporate |
| SOP Index | Kwality Labels INC - Corporate |
| Supplier Questionnaire - Addendum | Kwality Labels INC - Corporate |
| Supplier Questionnaire | Kwality Labels INC - Corporate |